is the hydrodynamic radius Rh does not describe
the effective radius of a hydrated molecule in solution as often mentioned.
It rather describes the radius of a hard sphere that diffuses at the same
rate as the molecule. The behavior of this sphere includes hydration and
shape effects. Since most molecules are not perfectly spherical, the Stokes
radius is smaller than the effective radius (or the rotational radius). A
more extended molecule will have a larger Stoke's Radii compared to a more
compact molecule of the same molecular weight. In liquids where there are
considerable interactions between solute and solvent molecule, Stokes radius
is proportional to frictional coefficient f and inverse proportional
to viscosity η:
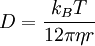
Frictional coefficient is determined by the size and
shape of the molecule under consideration.